While CDMOs were once seen as an alternative option before the pandemic, now many have come to view them as an essential part of the supply chain. They are even integral to helping drug developers stay focused on research and discovery.
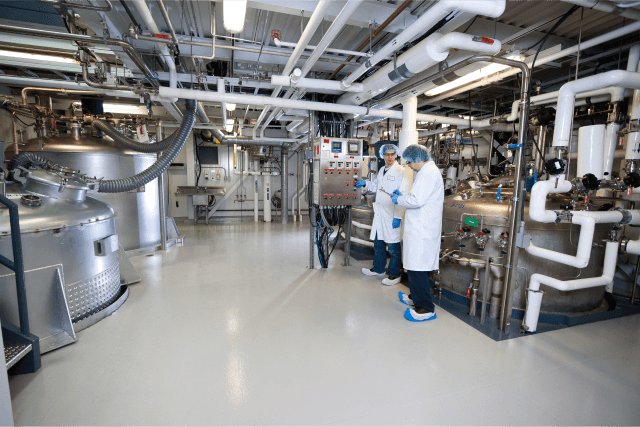
CDMO Samsung Biologics offers the most recent technologies with state-of-the-art CMO facilities to pharmaceutical companies and biotech companies. The CDMO provides the capabilities needed to assist pharmaceutical developers in manufacturing large batches of vaccines and treatments for patients as promptly and safely as possible.
Samsung Biologics provides drug substance and drug product CMO services for mammalian cell culture products adding mRNA vaccine to its business portfolio.
These services come with seamless quality from start to finish. They also include regulatory support with strong IP protection, dedicated project management to oversee the cycle of work required to support the client or partner, competitive pricing and flexible terms, and on time, in full delivery.
Partnership with Moderna
After a successful year of expansion, Samsung Biologics partnered with vaccine producer Moderna to support the production of hundreds of millions of doses of Moderna’s COVID-19 vaccine to markets outside of the U.S. Samsung Biologics will provide large-scale, commercial fill-finish manufacturing for Moderna’s mRNA-1273, the COVID vaccine that has already met with much success throughout the world.
“This vaccine is paramount to people around the world in the fight against the COVID-19 pandemic, and we truly appreciate our client Moderna for entrusting and choosing to partner with Samsung Biologics for the fill and finish of this important vaccine,” said Samsung Biologics CEO John Rim. “Due to the high level of urgency in supplying the vaccine to the global population, we have set immediate action plans and schedule to make mRNA-1273 available for commercial distribution in the early second half of 2021.”
With this business expansion, Samsung Biologics will work to grow its portfolio, create new business models, and add even more partnerships. In early 2023, the company will have finished building its fourth plant, which will have a bigger manufacturing capacity at a single site than any other plant.
The plant itself will work with the most up-to-date processes. Using Pharma 4.0-enabled technology, data integrity will be implemented throughout the system. It will also allow Samsung Biologics to work with multiple operation modes while still offering the best production efficiency. It will also be equipped with top-the-line, innovative biomanufacturing technologies.
Also see: Ghana’s environmental crisis, more than an emergency
Commitment to Environmental, Social & Corporate Governance
Even if a company has a reputation for being the best in the industry, it still has to challenge itself to do better. For Samsung Biologics, it isn’t just enough to offer quality CMO services. More must be done in order to make a lasting impact on the world and the world of medicine.
The company has made many efforts to commit to sustainability, including publishing its first-ever annual sustainability report. This report looked at the company’s past actions to provide socially responsible value to the global and local communities, as well as a ten-year plan to continue to address those goals.
CEO John Rim said, “The publication of our annual sustainability report showcases our aims to accelerate innovation and contribute to building a healthier future for all stakeholders. As we have always been since our founding in 2011, Samsung Biologics will continue to carry out its social accountability – creating a safer and healthier culture, reducing its environmental footprint, and increasing reporting transparency on ESG initiatives.”
Furthermore, the company added four more global ISO certifications. These included certifications in Business Community Management, Energy Management, Occupational Health, and Safety Management, and Effective Environmental Management.